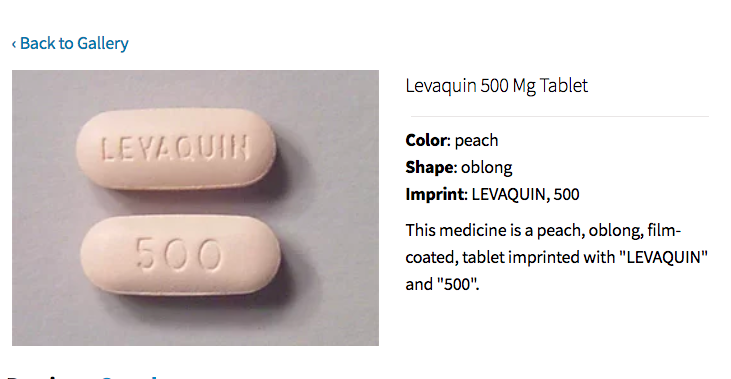
Janssen Pharmaceuticals, part of Johnson & Johnson, has stopped production of (brand-name) Levaquin, according to...
FDA Articles
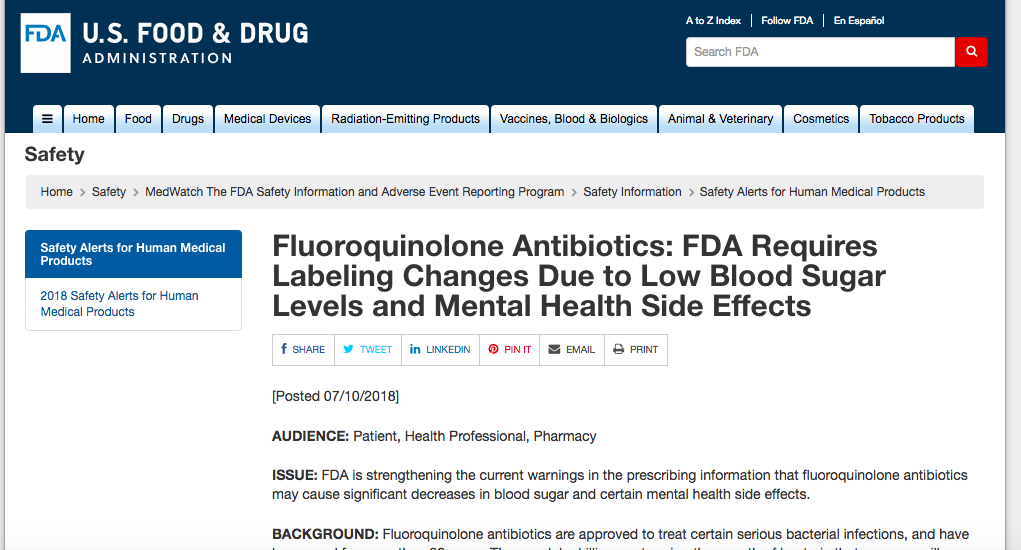
Fluoroquinolone Warning Labels Updated to Include Low Blood Sugar Levels and Mental Health Side Effects
On 7/10/18 the FDA announced that fluoroquinolone (Ciprofloxacin, Levofloxacin, Moxifloxacin, Ofloxacin, and a few...
Fluoroquinolones Increase Risk of Aortic Aneurysm
Evidence is mounting that fluoroquinolone antibiotics (Cipro, Levaquin, Avelox, Floxin, and their generic equivalents)...
Fluoroquinolone Warning Labels to be Updated in Canada
Health Canada, the department of the government of Canada with responsibility for national public health (like the...
Consumer Reports Warns Patients About Fluoroquinolone Dangers
Consumer Reports has published several articles about the dangers of fluoroquinolone antibiotics (including...
New Fluoroquinolones in the Pipeline
The most commonly prescribed fluoroquinolones are Cipro/ciprofloxacin, Levaquin/levofloxacin, Avelox/moxifloxacin, and...
Publicizing Fluoroquinolone Warnings
I have such mixed feelings about the FDA's response to the November, 2015 Antimicrobial Drugs Advisory Committee...
Updated Black-box Warnings for Fluoroquinolones
In July, 2016, the FDA made significant changes to the warning labels for all fluoroquinolone antibiotics...
Fluoroquinolones Removed From the Market
Several fluoroquinolones have been removed from the market because they caused acute toxicity and death. The...