I had the pleasure of interviewing Tamara for Episode 26 of The Floxie Hope Podcast. Please check out the podcast...
ciprofloxacin Articles
EMA Hearing on Fluoroquinolone Toxicity Part 1
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) held a hearing regarding...
Aortic Aneurysm and Dissection from Fluoroquinolones
This was originally sent to the people on the Floxie Hope Email List. If you would like posts like this sent to you,...
Getting My Life Back – Sreeji’s Story
I’ve been meaning to put down the insights I’ve gathered over the last six years for the benefit of others. So, here...
Can Floxies Drink Alcohol?
Many people have asked me if they can/should drink alcohol post-flox. As with most things, the answer is - it depends,...
Daniel W’s Recovery Story
Hi everyone! First off let me start by saying that I'm sorry you're here reading this story as chances are you're here...
Fluoroquinolone Antibiotics Increase Risk of Birth Defects
A few years ago, a friend from high school who was in her second trimester of pregnancy with her second child, reached...
Fluoroquinolones and Statins: A Recipe for Rhabdomyolysis
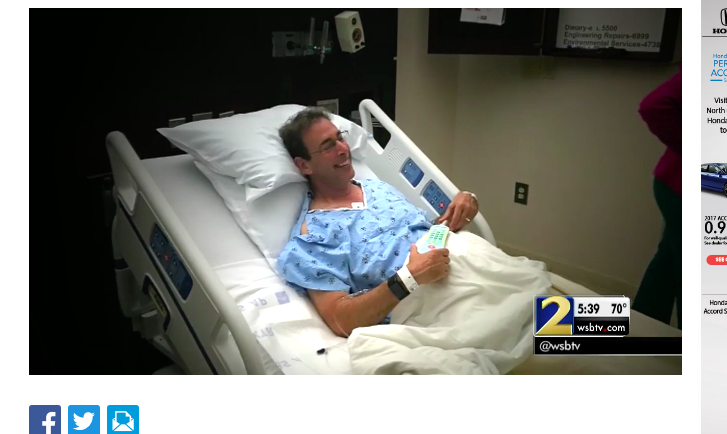
In May, 2017, WSB-TV 2 Atlanta aired the story, "Clark Howard says near-fatal disease possibly caused by popular...
Floxie Hope Podcast Episode 21 – James
James is featured in Episode 21 of The Floxie Hope Podcast. Check it out:...