What a difference a year makes I was “floxed” in June 2019 at the age of 44. I was prescribed a two-week course of...
ciprofloxacin Articles
Markus’s Story of Healing from Cipro Toxicity
It has been two years since I was floxed. I am forever grateful to the Floxie Hope creators and administrators for...
Patricia’s Story of Recovery from Cipro Toxicity
Summary Details: Story date: October 2019 (18 weeks after Cipro) Dose Taken: Cipro XR 1000 mg/day for 2 days (Total=...
Hazel’s Recovery Story: Visual System Damage from Avelox
Welcome to my Avelox Floxing story. Firstly, 1000 thanks to Lisa and all the contributors to Floxie Hope: your...
Madeline’s Story – Ciprofloxacin and Metronidazole Toxicity
Following is Madeline's story of her journey through ciprofloxacin and metronidazole toxicity. As you will see, her...
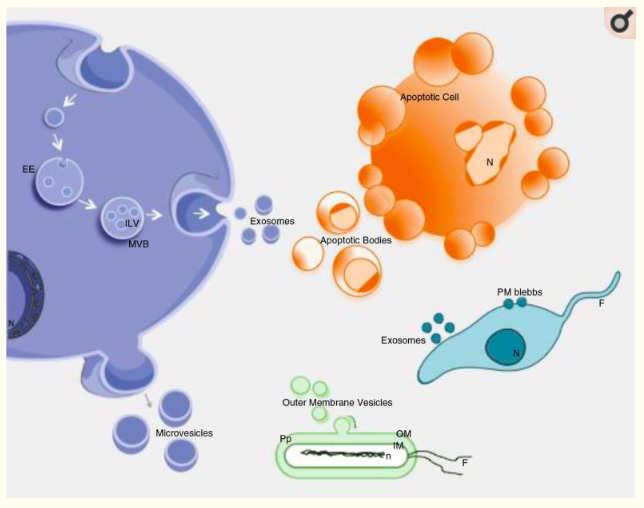
Ciprofloxacin Depletes Exosomal DNA
The study, "Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA"...
Emily’s Recovery Story – Cipro Toxicity
Here we are 11 weeks from me taking cipro ... In November 2018 I was given cipro to treat a UTI by my gynecologist. Me...
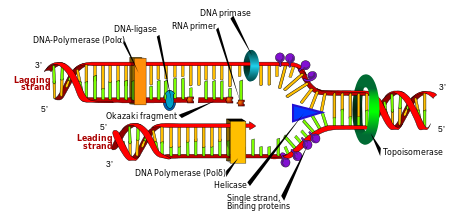
New Study Finds that Ciprofloxacin Depletes Mitochondrial DNA
An excellent article about the effects of ciprofloxacin (a fluoroquinolone antibiotic) on mitochondrial DNA was...
Cherise’s Story – Fluoroquinolone Toxicity, Faith, and Healing
Floxed 0 Faith 1 Who's keeping score? I AM.. I was prescribed ciploxx 500 x2 a day which is a fluoroquinolone...