First of all, to anyone who has shared their story or supported another Floxed, I want to thank you, from the bottom...
levofloxacin Articles
Trevor’s Story – Levofloxacin Poisoning and Recovery
In November of 2016 I was experiencing stomach issues. Typically I never go to the doctors, pretty much unless a bone...
Can Floxies Drink Alcohol?
Many people have asked me if they can/should drink alcohol post-flox. As with most things, the answer is - it depends,...
Floxie Hope Podcast Episode 24 – PJ
PJ shared his journey through fluoroquinolone toxicity on Episode 24 of The Floxie Hope Podcast. Check it out!...
Fluoroquinolones and Statins: A Recipe for Rhabdomyolysis
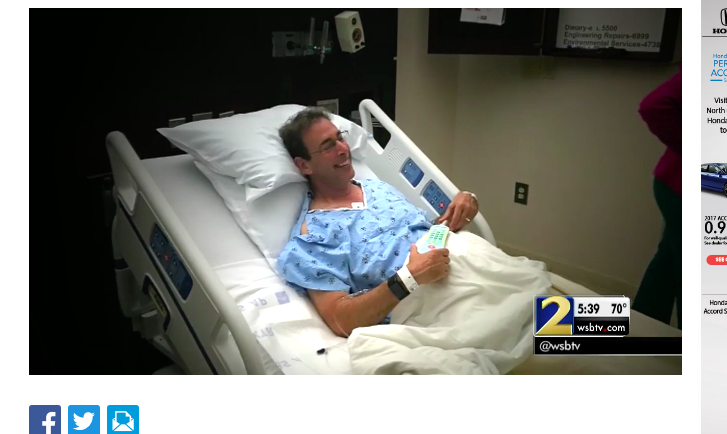
In May, 2017, WSB-TV 2 Atlanta aired the story, "Clark Howard says near-fatal disease possibly caused by popular...
Francesco’s Story – Recovery from Fluoroquinolone Toxicity
*The following is an individual's story of surviving fluoroquinolone toxicity. It is not medical advice. Please see...
Publicizing Fluoroquinolone Warnings
I have such mixed feelings about the FDA's response to the November, 2015 Antimicrobial Drugs Advisory Committee...
Fluoroquinolones Removed From the Market
Several fluoroquinolones have been removed from the market because they caused acute toxicity and death. The...
Can Fluoroquinolones Activate Mast Cells?
What is the connection between fluoroquinolone toxicity and mast cell activation / histamine intolerance? Can...