In 1992 the fluoroquinolone antibiotic Omniflox/temifloxacin was removed from the US market after causing three deaths.
To note the removal from the market, the FDA released the following statement:
“The Food and Drug Administration today announced that Abbott Laboratories of Abbott Park, Ill., is voluntarily recalling the broad-spectrum anti-infective drug Omniflox (temafloxacin) tablets, and will halt all further distribution of the drug.
This action is being taken because of severe adverse events associated with the use of the drug that have been reported to the company and to FDA in the first three months of marketing.
Temafloxacin was approved in late January 1992 and marketed in mid-February. Since that time there have been approximately 50 reports of serious adverse reactions, including three deaths. There were several cases of severe low blood sugar, especially in very elderly patients with decreased kidney function. Among the severe reactions there were a number of cases of an unusual complex of adverse reactions consisting of hemolitic anemia (destruction of red blood cells) and other blood cell abnormalities.”
I’m glad that Omniflox/temafloxacin was removed from the market. I’m glad that, in 1992, the FDA was able to move quickly to do the right thing and take a dangerous drug off the market.
It begs the question though – Why is Levaquin/levofloxacin still on the market?
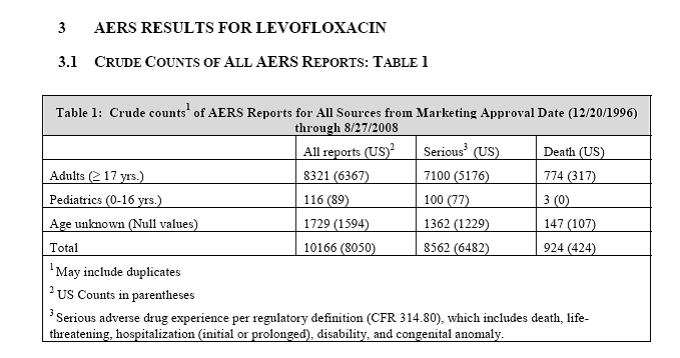
Levaquin/levofloxacin has caused far more than three deaths. According to an FDA review with the subject, “Pediatric Exclusivity Postmarketing Adverse Event Review,” between 12/20/1996 and 08/27/2008, 924 people were killed by Levaquin/levofloxacin, including three children.
It should be noted that, “Many studies have documented that only 10%-15% of serious adverse reactions are reported” to the FDA. Seeing as adverse reactions to Levaquin/levofloxacin, and other fluoroquinolone antibiotics, are often delayed and bizarre in their nature (who would think that multi-symptom, chronic illness could result from taking an antibiotic?), it is reasonable to think that only 10% of the reactions to Levaquin/levofloxacin are reported to the FDA.
Assuming that only 10% of the harm was reported, 9,240 people (including 30 children) were killed by Levaquin/levofloxacin in the 12 years measured.
Additionally, there were 10,166 reports of harm done by Levaquin/levofloxacin in that time period. If that’s 10% of the actual harm done, 101,660 people were harmed by Levaquin/levofloxacin in that 12 year period.
Again, THREE people died from Omniflox/temafloxacin before it was promptly pulled from the market in 1992. Johnson & Johnson is apparently on friendlier terms with the FDA than Abbott Labs.
Rest in Peace Chris Dannelly
Since 2008, Levaquin/levofloxacin has claimed many more victims. Chris Dannelly is one of the people who tragically died after taking levofloxacin in January, 2013. Chris was a 41 year old husband and father of two young kids. He was an athlete at the prime of his life.
Chris was killed by two pills of levofloxacin. You can read Chris’s story HERE. From Chris’s Story, “After reviewing the autopsy report, doctors stated that ‘all signs point to Levaquin’ as being the cause of death.” And, “Chris was a healthy, active man in the prime of his life. He worked out five days per week, and enjoyed playing both indoor and outdoor soccer regularly. Within a matter of less than a week’s time he went from being perfectly healthy, to losing his life…all because he took two little pills of Levaquin.”
Chris’s tragic story, highlighted by WSB-TV 2 out of Atlanta, Georgia, and told by Chris’s brave wife Kathy, can be viewed here –
Kathy states in the interview, “If I can get involved in this drug somehow coming off the market one day – then that’s the least I can do.”
Because Chris was given levofloxacin, the generic form of the drug, and the Supreme Court decided that drug manufacturers couldn’t be held responsible for harm caused by generic drugs, neither Kathy nor her children have the opportunity to pursue legal recourse for Chris’s tragic death. Taking this horrible drug that killed her husband off the market is the least the FDA can do to right this tragic situation of a husband, father and athlete being killed by a dangerous drug like Levaquin/levofloxacin.
Rest in Peace Richard Davis
Also on WSB-TV 2 Atlanta, Sandy Davis tells the story of how her husband Richard was killed by Levquin/levofloxacin:
Sandy Davis states, “I don’t think it should even be on the market. If it’s killing people – No.” Indeed. Richard was 60 years old when he passed.
But, but, but…
People will argue that we need Levaquin/levofloxacin because bacterial resistance to antibiotics is making more powerful antibiotics necessary. There may be some truth to that argument. But rather than seeing dangerous, complex drugs like Levaquin/levofloxacin in simplistic black and white terms, perhaps some thoughtful consideration of the grey area is in order.
IF it is deemed impossible for the FDA to remove Levaquin/levofloxacin from the market (and I do believe that it should be taken off the market – it is, after all, KILLING PEOPLE), it should be severely restricted. Levaquin/levofloxacin should ONLY be used in life or death situations. It should ONLY be used when all other non-fluoroquinolone antibiotic options have been exhausted. It should ONLY be used in patients who haven’t previously been exposed to fluoroquinolones. It should ONLY be used in people who are not genetically predisposed toward an adverse reaction to fluoroquinolones. (Who is genetically predisposed toward an adverse reaction to Levaquin/levofloxacin? Good question. Figure it out, FDA.) It should NEVER be given to children, athletes, the elderly, those with an autoimmune disease, those with autonomic nervous system dysfunction, those with a history of psychiatric illness, or anyone who needs to use contraindicated drugs, like steroids and NSAIDs, to survive.
IF Levaquin/levofloxacin must stay on the market, everyone who takes it should be provided with information that ensures that they know that Levaquin/levofloxacin can cripple or kill them.
Before anyone insists that Levaquin/levofloxacin must stay on the market, perhaps we (collectively) should do some due diligence to make sure that it, and other fluoroquinolone antibiotics, don’t have intergenerational adverse effects. After all, their mechanism of action is interruption of topoisomerase enzymes, enzymes that are necessary for DNA and RNA replication. In case it needs to be said, disrupting the DNA and RNA replication process of cells may have some deleterious effects on future generations. Don’t assume for a second that this concern has been examined. Warnings about the DNA toxicity of fluoroquinolone antibiotics have been ignored.
Though I understand that antibiotic resistance and loss of antibiotic efficacy is a problem, I can’t say that I entirely buy arguments that Levaquin/levofloxacin must stay on the market. No one claims that we are worse off because Omniflox/temafloxacin was removed from the market. Infections were also being adequately treated by non-fluoroquinolone antibiotics prior to 1987, when Levaquin/levofloxacin was approved by the FDA.
Kathy Dannelly and her children, Sandy Davis and her loved ones, and all other loved ones of victims of Levaquin/levofloxacin deserve justice. Many of them aren’t getting justice. The least that the FDA can do is keep others from dying in pain from fluoroquinolone toxicity caused by Levaquin/levofloxacin.













… [Trackback]
[…] Read More Information here to that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Info on that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Find More on that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Find More Information here on that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Read More Information here on that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Read More to that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Find More on that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Find More on that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Read More on to that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] Read More to that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]
… [Trackback]
[…] There you will find 40764 additional Info on that Topic: floxiehope.com/why-hasnt-levaquin-been-taken-off-the-market/ […]