Do you have a headache? Do you want one? If so, read this article –
“Mechanisms of Pathogenesis in Drug Hepatotoxicity Putting the Stress on Mitochondria” written by
Dean P. Jones, John J. Lemasters, Derick Han, Urs A. Boelsterli, Neil Kaplowitz
If you want a headache that lasts a while, read these articles that give background information as to why what is in “Mechanisms of Pathogenesis in Drug Hepatotoxicity Putting the Stress on Mitochondria” is important.
Drug Metabolism and Disposition, “Acyl Glucuronidation of Fluoroquinolone Antibiotics by the UDP-Gulucuronosyltransferase 1A Subfamily in Human Liver Microsomes”
and
Current Drug Metabolism, “Acyl Glucuronides: Mechanistic Role in Drug Toxicity?”
Despite their headache inducing capabilities, the articles are actually quite interesting and important. To highlight how and why they are important, here is my breakdown of “Mechanisms of Pathogenesis in Drug Hepatotoxicity Putting the Stress on Mitochondria.” As I have done with other journal articles, I have taken quotes from the article and commented under them.
“Mitochondrial impairment is usually a final event common to pathways leading to necrotic and apoptotic cell death.”
Since mitochondrial impairment leads to cell death, perhaps it would be nice for the FDA to examine how pharmaceuticals affect mitochondria before approving them. Sadly, they don’t think so, as “mitochondrial toxicity testing is still not required by the US FDA for drug approval.” (http://psychrights.org/Research/Digest/NLPs/DrugsCauseMitochondrialDamage.pdf)
“it is important to consider whether drug-induced participation of mitochondria in hepatocellular death is a direct result of drugs acting on these organelles (e.g., drug accumulation, inhibition of electron transport and fatty acid oxidation, or depletion of anti-oxidant defense) or an indirect result ensuing from mitochondrial participation in programs of cell death.”
They found that both were the case. This stuff is very complex and not linear. One reaction causes another reaction, on and on for a while. It’s not an either/or situation.
“Oxidative stress is often defined as an imbalance of pro-oxidants and antioxidants; however, the finding that thiols [i.e., glutathione (GSH) and cysteine (Cys)] in plasma are not in redox equilibrium with their disulfide products [i.e., respectively, GSSG and CySS] (1, 2) and that their plasma concentrations are substantially displaced from cellular values (3) has significantly altered concepts of oxidative stress (4, 5). For example, the in vivo “balance” of pro-oxidants and antioxidants cannot be defined by any single entity, such as an equilibrium constant, and our growing knowledge of signaling mechanisms indicates that oxidative stress may be better defined as a disruption of redox signaling, rather than as an imbalance of pro-oxidants and antioxidants. The failure of large-scale, double-blind interventional trials with free-radical scavenging antioxidants may likewise reflect an oversimplified therapeutic approach.”
If you have too many oxidants/oxidative stress in your system, you should just add antioxidants to restore the balance of oxidants and antioxidants, right? Well, it’s not that simple. Once the signaling mechanisms within mitochondria start the process of oxidative stress and apoptosis (programmed cell death), you can’t stop the process or repair the damage by adding more antioxidants to the mix. If it was as simple as a disruption in the balance between oxidants and antioxidants, we would all be cured by glutathione drips and vitamin C supplements. Unfortunately, there are complex feedback loops that make the process much more difficult to fix than that. I’m not saying that glutathione drips and vitamin C supplements aren’t worth a try, it’s just that adding antioxidants to make up for the excess of oxidative stress (in the form of Reactive Oxygen Species and Reactive Nitrogen Species) is an oversimplified approach.
“Cells that overexpress the mitochondrion-specific thioredoxin Trx2, however, have been found to be resistant to tBH-induced loss of mitochondrial membrane potential and apoptosis”
Mitochondrion-specific thioredoxin Trx2 (http://en.wikipedia.org/wiki/Thioredoxin) is protective. I’m not sure exactly what the implications of this are, but I suspect that those of us who got “floxed” have low levels of mitochondrion-specific thioredoxin Trx2. (We probably are deficient in cellular magnesium and have a genetic predisposition toward susceptibility to mitochondrial injury and oxidative stress too.)
“Mitochondrial Trx2 responds to changes in the extracellular redox potential of Cys/CySS (http://en.wikipedia.org/wiki/Cysteine) (EhCys/CySS) over a range that is relevant to cardiovascular disease in humans.” and “Previous in vitro findings support a cause–effect relationship for plasma CySS in cell signaling pathways associated with cardiovascular disease.”
Cardiovascular disease is related to mitochondrial function and oxidative stress / antioxidants.
Every chronic disease that plagues humans has its roots in mitochondrial dysfunction. That may be Lisa’s theory, or it may be the truth. TBD. But there are enough journal articles noting how mitochondria relate to all sorts of chronic diseases that you’d think that our regulatory agencies would require that the effects of pharmaceuticals on mitochondria be tested before they are released to the market. But no, they don’t. They’re incompetent fools. And because of their foolishness the pharmaceutical companies really have gotten away with creating customers, not cures.
“Mass spectrometry-based redox proteomics show that several classes of plasma membrane and cytoskeletal proteins involved in inflammation respond to this redox switch (Trx2), including vascular cell adhesion molecule, integrins, actin, and several Ras family GTPases.”
This really cryptic, difficult to understand sentence may actually say a lot about FQ toxicity. The Trx2 redox switch (http://en.wikipedia.org/wiki/Thioredoxin) is protective against loss of mitochondrial membrane potential and apoptosis (see above). So, perhaps underexpression of the Trx2 redox switch leads to inflammation of vascular cell adhesion molecules, integrins, actin, and several Ras family GTPases. What are these things, you ask? Wiki will tell us!
Vascular Cell Adhesion Molecules – http://en.wikipedia.org/wiki/VCAM-1 – “The VCAM-1 protein mediates the adhesion of lymphocytes, monocytes, eosinophils, and basophils to vascular endothelium. It also functions in leukocyte-endothelial cell signal transduction, and it may play a role in the development of atherosclerosis and rheumatoid arthritis.” (I’ll let you look up all of the words you don’t know in this – ugh.)
Integrins – http://en.wikipedia.org/wiki/Integrin and http://www.cs.stedwards.edu/chem/Chemistry/CHEM43/CHEM43/CellAdhesion/integrinfunction.htm – “Integrins are cell-surface receptors that mediate cell-cell adhesion and are of great importance in binding and interactions of cells with components of the extracellular matrix (ECM) such as fibronectin (and cell-matrix). Importantly, integrins facilitate “communication” between the cytoskeleton and extracellular matrix, allowing each to influence the orientation and structure of the other.” When your integrins are messed up, your cytoskeleton can get messed up. Or something like that.
Here is an about how fluoroquinolones that mentions how they relate to integrins –
http://intl-vet.sagepub.com/content/38/2/143.full – “Lack of extracellular Mg2+ impairs the function of integrins. These transmembrane proteins connect the cells to extracellular matrix” This stuff has something to do with how fluoroquinolones mess up tendons. Yeah.
Actin – http://en.wikipedia.org/wiki/Actin It’s important for cellular function. (That’s all I’ve got for you – read the wiki :p )
Ras Family GTPases – http://en.wikipedia.org/wiki/GTPase It’s important for cellular function. (That’s all I’ve got for you – read the wiki :p )
You could get completely lost looking up all of the different systems described within this sentence. Now that I have dug through it, maybe the authors of the study stated things as succinctly as possible. This stuff is hard.
If someone significantly smarter than me wants to figure out how each of these cellular functions relate to magnesium, and, of course, floxing, that would be great.
Chelatable Iron, Oxidative Stress, and Cell Death
This whole section is about how iron relates to drug induced liver injury (DILI). I’m not going to go over it piece by piece. One thing that makes me curious about this section is that iron helped me to feel better than any other supplement. I wonder why that is. If the answer is in the article, I don’t understand chemistry well enough to get it from the article.
“In the mitochondrial permeability transition (MPT), high-conductance permeability transition (PT) pores open that make the mitochondrial inner membrane nonselectively permeable to all solutes of molecular mass up to approximately 1500 Da (59, 60). Calcium ion, oxidative stress, and numerous reactive chemicals induce onset of the MPT, whereas cyclosporin A (CsA) and pH less than 7 inhibit pore opening. After MPT onset, mitochondrial depolarize and undergo large-amplitude swelling driven by colloid osmotic forces, which are the hallmarks of the MPT. Swelling leads to rupture of the mitochondrial outer membrane and release of proapoptotic cytochrome c and other factors from the intermembrane space.”
Calcium + oxidative stress = apoptosis. I’ve seen this elsewhere – https://floxiehope.com/2013/12/17/article-breakdown-mitochondrial-reactive-oxygen-species-control-t-cell-activation-by-regulating-il-2-and-il-4-expression-mechanism-of-ciprofloxacin-mediated-immunosuppression/
Interplay of Signal Transduction and Mitochondria in the Acetaminophen model
This section goes over how acetaminophen causes mitochondrial damage and drug induced liver injury. It’s not a one-step process – it’s really complex and multiple things have to go wrong, at a cellular level, at once. But it can happen.
As I mentioned above, I hypothesize that pharmaceutical induced mitochondrial injury is the cause of most chronic diseases. Per Dr. Richard Boles, an expert in mitochondrial dysfunction and diseases:
“these are partial defects. Mitochondrial dysfunction doesn’t really cause anything, what it does is predisposes towards seemingly everything. It’s one of many risk factors in multifactorial disease. It can predispose towards epilepsy, chronic fatigue, and even autism, but it doesn’t do it alone. It does it in combination with other factors, which is why in a family with a single mutation going through the family, everyone in the family is affected in a different way. Because it predisposes for disease throughout the entire system.” (http://www.hormonesmatter.com/cyclic-vomiting-syndrome-mitochondrial-dysfunction/)
Is acetaminophen causing mitochondrial damage??? Is it damaging or depleting mtDNA? Is that damage hereditary???? Because if ACETAMINOPHEN is leading to a variety of chronic diseases, ugh, well, we might just be fucked (sorry, I couldn’t think of another word for the situation).
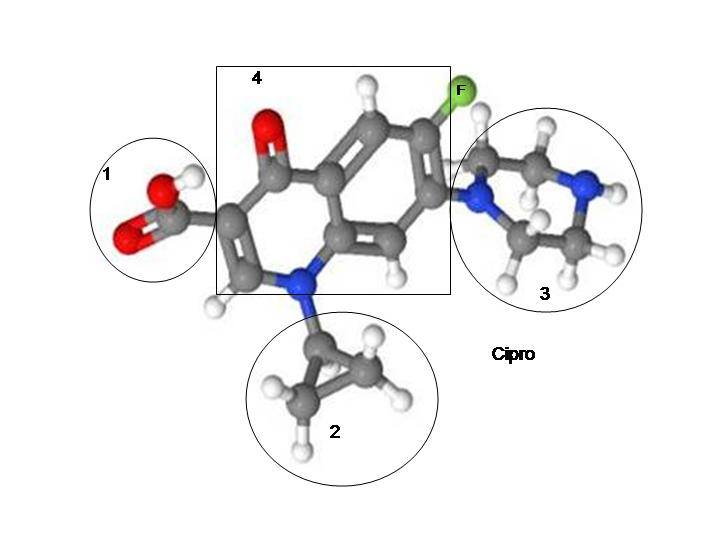
Fluoroquinolones deplete mitochondrial DNA content – “Interestingly, as an inhibitor of bacterial topoisomerase II and an inducer of DNA double-strand breaks, ciprofloxacin was also shown to deplete the mitochondrial DNA (mtDNA) content, thus leading to mitochondrial dysfunction and retarded cellular growth (15–17).” http://www.jimmunol.org/content/184/9/4827.full.pdf Awesome, huh?
I think that fluoroquinolone induced mitochondrial damage, both direct and hereditary, is responsible for the increase in every chronic disease that has increased in prevalence along with fluoroquinolone use.
“One of the most striking and puzzling clinical hallmarks of idiosyncratic (host-dependent) DILI is the delayed onset of the disease. In fact, the time between initiation of daily drug treatment and the presentation of biochemical markers and clinical symptoms of liver injury can vary from a few weeks to several months, sometimes even exceeding a year (89). The reason for the long lag, often followed by an abrupt progression to DILI, is currently not known. However, it is clear, for the vast majority of drugs, that the delayed time to onset is not related to a gradual accumulation (of drug or drug metabolite) that would eventually lead to critical and toxicologically relevant concentrations in the liver. Instead, the lag time could be explained by an accumulating effect of a drug. This notion, together with experimental findings, is in line with the concept that mitochondria are involved in the etiology of DILI, because damage to mitochondria often reflects successive chemical insults, such that no immediate cause for functional changes or pathological alterations can be established. There is indeed experimental evidence that prolonged injury to mitochondria, such as that which typifies oxidative injury to mitochondrial DNA or to components of the electron transport chain (ETC), has to cross a certain threshold (or a number of thresholds) before cell damage or cell death becomes manifest (Figure 4A).”
Underlining added by Lisa. This paragraph explains both delayed reactions and the fact that most people have a tolerance threshold for fluoroquinolones. If your doctor, or anyone else, tells you that your reaction that came months after you stopped administration of a fluoroquinolone “couldn’t have happened because of the FQ, because it was metabolized already,” or something like that, tell him or her to read this paragraph as many times as it takes to understand it. Damage to mitochondria, whether related to DILI or not, is not linear and it is not (necessarily) immediate. Unfortunately, this is not understood by anyone other than the authors of this study, and probably a few other scientists, so suing based on a delayed reaction to a drug that you have tolerated well in the past is difficult to impossible.
“This non-linear response can be explained upon consideration that the molecules that subserve mitochondrial function (e.g., mitochondrial DNA, mRNA, and ETC proteins) are present in excess of amounts required for normal cell function. This reserve (or buffering) capacity acts as a protective mechanism; however, at a certain stage of damage, the supply of biomolecules needed to support wild-type mitochondrial function becomes compromised.”
For each of us Floxies, the reserve capacity of our mtDNA has been depleted. I have no clue if it can be built up again or not.
“a number of human mitochondrial genetic diseases that are clinically discreet are being diagnosed at unexpected rates”
It is REALLY IMPORTANT that it be determined whether or not pharmaceutical induced damage to mitochondria is hereditary. Seriously scientists – important stuff – answers will be greatly appreciated.
“First, all the investigated drugs (including trovafloxacin, a fluoroquinolone) invariably decreased the activity of key mitochondrial proteins that are sensitive to oxidant stress (e.g., aconitase-2, complex I) and often decreased the expression of mitochondrial (but not nuclear) genes (120). Second, we found that these markers of mitochondrial injury became apparent only after four weeks, although a number of cytoprotective pathways were activated within two weeks. It thus appears that an initial adaptive response was followed by a toxic response (121), possibly also involving a threshold.”
What happens when expression of mitochondrial genes are decreased? What are the implications of this finding?
The fact that there is an initial adaptative response followed by a toxic response (to pharmaceutical induced mitochondrial injury) may explain why there are so many different results to studies of fluoroquinolones (and other mito damaging drugs). Long-term studies need to be done. Studies that take into consideration that delayed reactions occur, need to be done. Studies that take into consideration tolerance thresholds need to be done. Please.
“First, superoxide that escapes dismutation to hydrogen peroxide cannot cross the inner mitochondrial membrane and can oxidize [Fe-S]-containing enzymes (e.g., aconitase and complex I/III subunits). Alternatively, superoxide can rapidly react with mitochondrial nitric oxide (NO) to form peroxynitrite (ONOO−). For example, the fluoroquinolone antibiotic trovafloxacin (TVX), a typical DILI-associated drug, raises steady-state levels of NO in hepatocellular mitochondria (unpublished data). The mechanisms are not known, but TVX also increases cytosolic (non-ferritin-bound) Ca2+, likely activating the Ca2+-dependent mitochondrial NO synthase (123) to produce ONOO−. Peroxynitrite is dangerous for a number of reasons: i) under acidic conditions, it can be degraded to form the extremely reactive hydroxyl radical; ii) it may directly cause the nitration of aconitase, Sod2, and the [Fe-S]-containing subunits of ETC complexes; and iii) it can induce mitochondrial permeabilization (Figure 4B) (124). This superimposed oxidative/nitrative stress could ultimately push the cell across the threshold to observable injury.”
Floxies – that’s what happened to you (and me). It’s really hard to understand, I know. I have a headache right now and I’m guessing that you do too if you’ve gotten this far in the post. It’s important information though.
On a light note, I think that it’s funny that fluoroquinolones convert “NO” into “ONOO” in our cells. Yup, that’s about what it feels like – “no” turning into “oh no” turning into “oh fuck” which turns into “fuck you Bayer / Johnson & Johnson.” 🙂
The end of the article and my comments.
Dean P. Jones, John J. Lemasters, Derick Han, Urs A. Boelsterli and Neil Kaplowitz are brilliant. I thank them very much for this article. It answers a lot of questions. It still leaves many unanswered, of course – as any good article does. I hope that they, and more scientists, are doing more work on the relationship between pharmaceutical induced mitochondrial injury and disease states.










It may be the end of your article Lisa , but I can promise you that It will never, as long as I have breath in my body, be the end of my fight to get these lethal poisons recognised for what they actually are. And when / if, eventually I can no longer fight , if god help us this has STILL not by then been achieved, then on the word of my Children, who have promised me they then will continue my battle, this fight will be passed down to future generations for as long as It takes, to help expose those who are responsible for this crime against humanity & to get them to answer for this atrocity, that is simply nothing, but cold blooded legalised MURDER .
I had this instinct to avoid Tylenol too… glad to see it validated somewhat. You’re right though, this is hard to follow, especially with fatigue and brain fog lol. Read the sentence 10 times then give up and move on to the next.
Really nice info here though, especially the delayed reaction, which I hope not to experience and wish to prevent somehow (mine was almost immediate, and am assuming it was the cipro at this point, at least the musculo; nothing else has come forward). But definitely bookmarking this for reference. Wish had something to add.
Ask and ye shall recieve: “Most significantly, the study demonstrated that PQQ not only protects mitochondria from oxidative stress—it promotes the spontaneous generation of new mitochondria within aging cells, a process known as mitochondrial biogenesis.” http://en.wikipedia.org/wiki/Pyrroloquinoline_quinone
also found here, http://www.lef.org/magazine/mag2010/ss2010_Rejuvenate-Your-Cells-Growing-New-Mitochondria_01.htm, is a quote worth paying attention to: “Up until now, the best we could do was protect and improve the function of existing mitochondria using nutrients like L-carnitine, lipoic acid, and coenzyme Q10.”
Greetings from Florida! I’m bored to tears at
work so I decided to browse your site on my iphone during lunch break.
I love the information you present here and can’t wait to take a look when I get home.
I’m surprised at how quick your blog loaded on my cell phone
.. I’m not even using WIFI, just 3G .. Anyways, superb site!
Did you happen to read this one which sites the article above? https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5085033/
Regarding PQQ, referenced by Ken: I think it helped me as I noticed a difference in my body when taking it verses not. I used Jarrow CoQ10 with PQQ.