For the FDA’s November 5, 2015 meeting to review “The Benefits and Risks of Systemic Fluoroquinolone Antibacterial Drugs for the Treatment of Acute Bacterial Sinusitis (ABS), Acute Bacterial Exacerbation of Chronic Bronchitis in Patients Who Have Chronic Obstructive Pulmonary Disease (ABECB-COPD), and Uncomplicated Urinary Tract Infections (uUTI)” a 617 page report was released by the FDA. You can access it HERE if you want to read it in its entirety.
In the last post, I noted that the FDA report said that fluoroquinolones have not been shown to be any better than a placebo at treating sinus infections, bronchitis in those with COPD, or uncomplicated urinary tract infections. In this post, I will point out that the FDA has given those suffering from fluoroquinolone toxicity an official name. Per the report:
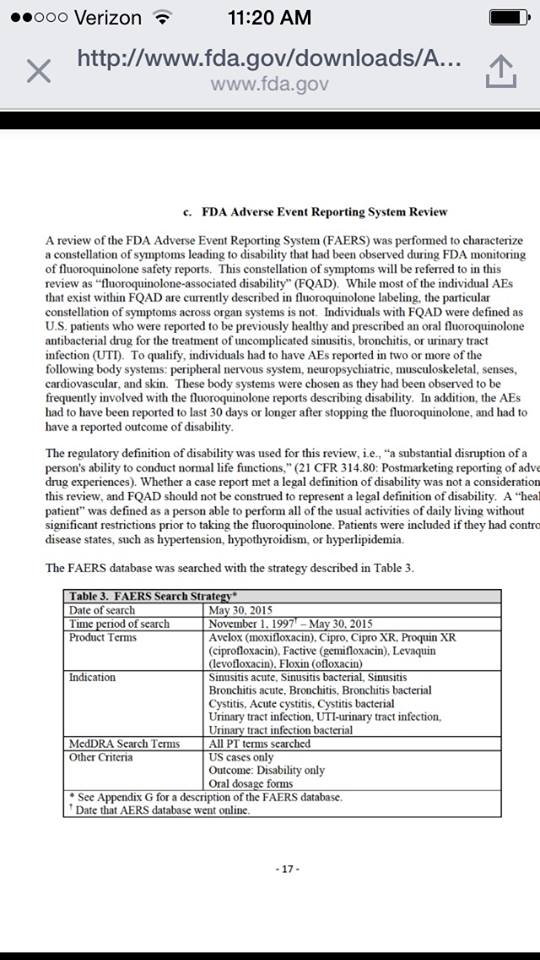
“A review of the FDA Adverse Event Reporting System (FAERS) was performed to characterize a constellation of symptoms leading to disability that had been observed during FDA monitoring of fluoroquinolone safety reports. This constellation of symptoms will be referred to in this review as ‘fluoroquinolone-associated disability’ (FQAD). While most of the individual AEs that exist within FQAD are currently described in fluoroquinolone labeling, the particular constellation of symptoms across organ systems is not. Individuals with FQAD were defined as U.S. patients who were reported to be previously healthy and prescribed an oral fluoroquinolone antibacterial drug for the treatment of uncomplicated sinusitis, bronchitis, or urinary tract infection (UTI). To qualify, individuals had to have AEs reported in two or more of the following body systems: peripheral nervous system, neuropsychiatric, musculoskeletal, senses, cardiovascular and skin. These body systems were chosen as they had been observed to be frequently involved with the fluoroquinolone reports describing disability. In addition, the AEs had to have been reported to last 30 days or longer after stopping the fluoroquinolone, and had to have a reported outcome of disability.”
That recognition from the FDA is EXCELLENT progress!
I don’t know whether or not FQAD will be put into diagnostic manuals, or if it will be coded for in insurance systems. I hope that those are future steps that will be taken.
So far, in the first 20 pages of the 617 page FDA report, they have noted that fluoroquinolones are no more effective than placebos in treatment of sinus infections, bronchitis in those with COPD, and uncomplicated urinary tract infections. They have also noted that fluoroquinolones can cause a constellation of symptoms across multiple body systems, and that those symptoms can lead to disability.
It is not appropriate to cause, or even to risk, disabling adverse effects through utilization of a drug that is no more effective than a placebo at treating sinus infections, bronchitis in those with COPD, and uncomplicated urinary tract infections. I hope that the FDA changes the recommended uses for fluoroquinolones in recognition of this.
I hope that the naming of FQAD increases recognition of the horrible adverse effects of fluoroquinolones. With recognition, hopefully a more prudent and appropriate approach to use of fluoroquinolones will occur.
Post-publishing edit – While it is a wonderful step in the right direction that the FDA acknowledged that fluoroquinolones can cause a constellation of symptoms that is not adequately noted in the warning label, I may have jumped the gun a bit in calling it an “official” name. FQAD is the term that the FDA is using for the purposes of the November 5th hearing. It is not a diagnostic code that your doctor can look up in his or her diagnostic manuals yet. I hope that it’s a step in that direction, but we’re not there yet. Celebrating the FDA acknowledgement is in order, but we still have a ways to go. I apologize for not being more clear in the post before I originally published it!









… [Trackback]
[…] Find More Info here on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Find More Info here to that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Read More Info here on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Read More here to that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] There you can find 16958 more Info to that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Find More Info here on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Find More on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Read More to that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Find More Information here on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Find More on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Information on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]
… [Trackback]
[…] Read More Info here on that Topic: floxiehope.com/an-official-name-fluoroquinolone-associated-disability-fqad/ […]