For the FDA’s November 5, 2015 meeting to review “The Benefits and Risks of Systemic Fluoroquinolone Antibacterial Drugs for the Treatment of Acute Bacterial Sinusitis (ABS), Acute Bacterial Exacerbation of Chronic Bronchitis in Patients Who Have Chronic Obstructive Pulmonary Disease (ABECB-COPD), and Uncomplicated Urinary Tract Infections (uUTI)” a 617 page report was released by the FDA. You can access it HERE if you want to read it in its entirety.
In the next several posts, I will summarize and comment on the report. The following is post 1 of the summary/commentary:
The report starts by noting that fluoroquinolones have never been shown to be safe or effective treatments for sinusitis, bronchitis for those with COPD, or uncomplicated urinary tract infections. No placebo-controlled trials were conducted when approving fluoroquinolones as treatments for these diseases. Per the FDA:
“Antibacterial drugs approved before the 1980s were in general used as the control antibacterial drugs in NI trials. Because placebo-controlled trials were not used as a basis for the approval of those drugs, a treatment effect of the control antibacterial drugs over placebo had not been clearly established for ABS, ABECB-COPD, or uUTI. Thus, these active-controlled studies may not provide a reliable means to evaluate efficacy of antibacterial drugs for these indications.”
No placebo-controlled studies were used for approval of fluoroquinolones (or any other antibacterial drugs, apparently) in the treatment of sinus infections, bronchitis for those with COPD, or uncomplicated urinary tract infections. It’s just now occurring to the FDA that without placebo-controlled trials, they may not have reliable evidence of the efficacy of these drugs. Ya think?
The report goes on to note that the Cochrane Collaboration concluded the following about treatment of sinusitis with antibiotics:
“The Cochrane Collaboration conducted a review of antibacterial drugs for treatment of clinically diagnosed acute rhinosinusitis in adults and provided this statement in their conclusion: ‘Taking into account antibiotic resistance and the very low incidence of serious complications, we conclude that there is no place for antibiotics for the patient with clinically diagnosed, uncomplicated acute rhinosinusitis’ (Lemiengre, van Driel, et al, 2012).”
Additionally, regarding bronchitis in those with COPD, the FDA report notes that, “Clinical practice guidelines for treatment of ABECB-COPD published by the American College of Physicians stated, ‘Among patients with mild attacks, there were no significant differences between those who received antibiotics and those who received placebo.’”
The case regarding uncomplicated urinary tract infections is a bit more convoluted, but the report does note that, “In a study (of patients with uncomplicated urinary tract infections) that used ibuprofen as a control, there was no treatment difference on symptom resolution in comparison to an antibacterial drug.”
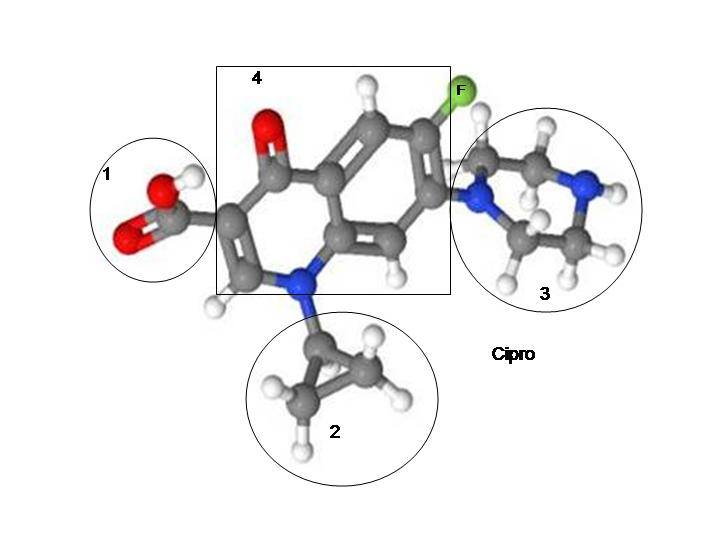
We rely on the FDA to evaluate drugs to make sure that they are safe and effective. Fluoroquinolones are far from safe—they lead to multiple musculo-skeletal and nervous system adverse effects (most of which are described in the 43 page warning label for Cipro/ciprofloxacin). I was assuming that they were at least effective. It turns out that fluoroquinolones are no more effective than a placebo at treatment of sinus infections, bronchitis or uncomplicated urinary tract infections. (They’re no more effective than a placebo at treating prostatitis either – see THIS POST.) People are getting poisoned and severely hurt by fluoroquinolones–for no good reason. Fluoroquinolones are no more effective than a placebo at treating many of the conditions they are prescribed for, and people would be far better off taking a sugar-pill than they are taking a topoisomerase interrupting chemo drug that is no more effective than a placebo.
The FDA approved fluoroquinolones for use in treatment of sinus infections, bronchitis for those with COPD, and uncomplicated urinary tract infections based on the assumption that they were safe and effective, not on actual studies showing that assumption to be true. They are not safe, and they are no better than a placebo for treatment of these conditions. For more than 30 years, the FDA has been using false assumptions and faith, rather than evidence established by placebo-controlled trials, as the basis for approving dangerous drugs for treatment of benign infections that the body can fight off using its immune system (or a placebo).
The mantra of “all drugs have side-effects” is often spewed by people who think that side-effects are acceptable. But with any veil of acceptability comes the assumption that dangerous drugs are at least effective. Fluoroquinolones aren’t even effective at treating sinusitis, bronchitis or uncomplicated UTIs—diseases that they are prescribed for thousands of times every day. They are no better than a placebo at treating those conditions. They are neither safe nor effective and people would be better off taking snake oil than they are taking ineffective drugs that deplete mitochondrial DNA and lead to tendon ruptures, permanent peripheral neuropathy, serious central nervous system adverse effects, and more.
Fluoroquinolones are neither safe nor effective. Every person who has been hurt by a fluoroquinolone taken to treat sinusitis, bronchitis or an uncomplicated UTI was hurt because of the FDA’s ineptitude and their inability to realize that antibiotics need to go through placebo-controlled trials just like every other drug.
This situation, where the FDA approves unsafe and ineffective snake-oils to be sold by the pharmaceutical juggernauts as long as they are labeled as “antibiotics,” is only going to get worse with the passage of the 21st Century Cures Act. The 21st Century Cures Act will encourage the production of new antibiotics, regardless of their safety profile or mechanism of action. In an op/ed article in the New England Journal of Medicine, it is noted that:
“The proposed legislation would make immediate changes with respect to new antibiotics and antifungals by enabling their approval without conventional clinical trials, if needed to treat a ‘serious or life-threatening infection’ in patients with an ‘unmet medical need.’ In place of proof that the antimicrobial actually decreases morbidity or mortality, the FDA would be empowered to accept nontraditional efficacy measures drawn from small studies as well as ‘preclinical, pharmacologic, or pathophysiologic evidence; nonclinical susceptibility and pharmacokinetic data, data from phase 2 clinical trials; and such other confirmatory evidence as the secretary [of health and human services] determines appropriate to approve the drug.’ Antimicrobials approved in this manner would carry disclaimers on their labeling, but there is no evidence that such a precaution would restrict prescribing to only the most appropriate patients. If passed in its current form, the bill would also provide hospitals with a financial bonus for administering costly new but unproven antibiotics, which could encourage their more widespread use. The bill gives the secretary of health and human services the authority to expand this nontraditional approval pathway to other drug categories as well, if “the public health would benefit from expansion.”
Don’t think for a second that the FDA is keeping snake-oils off the market. They’ve been allowing drugs that are more dangerous than snake-oils, and no more effective, to be sold to the American public for years.
I hope that they at least try to undo some of the damage done in the November 5th meeting. We shall see.










… [Trackback]
[…] Information on that Topic: floxiehope.com/the-fda-notes-that-fluoroquinolones-are-no-better-than-placebos/ […]
… [Trackback]
[…] Find More Information here to that Topic: floxiehope.com/the-fda-notes-that-fluoroquinolones-are-no-better-than-placebos/ […]
… [Trackback]
[…] Here you will find 90566 more Info on that Topic: floxiehope.com/the-fda-notes-that-fluoroquinolones-are-no-better-than-placebos/ […]
… [Trackback]
[…] Info to that Topic: floxiehope.com/the-fda-notes-that-fluoroquinolones-are-no-better-than-placebos/ […]
… [Trackback]
[…] Find More to that Topic: floxiehope.com/the-fda-notes-that-fluoroquinolones-are-no-better-than-placebos/ […]
… [Trackback]
[…] Read More to that Topic: floxiehope.com/the-fda-notes-that-fluoroquinolones-are-no-better-than-placebos/ […]
… [Trackback]
[…] Find More Information here on that Topic: floxiehope.com/the-fda-notes-that-fluoroquinolones-are-no-better-than-placebos/ […]