Fluoroquinolones are Mito-Toxic
Many articles about how fluoroquinolones damage mitochondria and lead to mitochondrial dysfunction have been published. In Science Translational Medicine, “Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells,” it is noted that bactericidal antibiotics, including ciprofloxacin, a fluoroquinolone, “damage mammalian tissues by triggering mitochondrial release of reactive oxygen species (ROS).” Additionally, in Molecular Pharmacology, “Delayed Cytotocicity and Cleavage of Mitochondrial DNA in Ciprofloxacin Treated Mammalian Cells” it is noted that fluoroquinolones “cause a selective loss of mitochondrial DNA (mtDNA)” and “The loss in mtDNA was associated with a delayed loss in mitochondrial function.” Even the FDA acknowledges that fluoroquinolones cause mitochondrial damage. In their April 27, 2013 Pharmacovigilance Review, “Disabling Peripheral Neuropathy Associated with Systemic Fluoroquinolone Exposure,” the FDA notes that the mechanism for action through which fluoroquinolones induce peripheral neuropathy is mitochondrial toxicity. The report says:
“Ciprofloxacin has been found to affect mammalian topoisomerase II, especially in mitochondria. In vitro studies in drug-treated mammalian cells found that nalidixic acid and ciprofloxacin cause a loss of mitochondrial DNA (mtDNA), resulting in a decrease of mitochondrial respiration and an arrest in cell growth. Further analysis found protein-linked double-stranded DNA breaks in the mtDNA from ciprofloxacin-treated cells, suggesting that ciprofloxacin was targeting topoisomerase II activity in the mitochondria.”
Fluoroquinolones are very, very bad for mitochondria. And, as the engines of our cells, healthy mitochondria are very necessary for healthy cells. Mitochondrial dysfunction is connected with many chronic diseases, including autism, CFS/ME, fibromyalgia, Alzheimer’s Disease, Parkinson’s Disease, multiple sclerosis, etc.
Even though there is quite a bit of evidence that fluoroquinolones damage mitochondria, it is not generally acknowledged that fluoroquinolones are mito-toxic. A petition has been filed with the FDA asking them to add a warning about mitochondrial toxicity to the label of fluoroquinolones. If the FDA adds the information that is in their internal documents to the public warning label, it will become accepted and acknowledged that fluoroquinolones damage mitochondria, and that fact will be further reflected in research articles. The ball has to start rolling somewhere. I thank the researchers who have uncovered the mitochondrial toxicity of fluoroquinolones very much for their research that got the realization of the mito-toxicity of fluoroquinolones started.
Similarities between Fluoroquinolones and other Mito-Toxic Drugs
A drug that is acknowledged to be mito-toxic is adriamycin (doxorubicin). In Toxicological Sciences, “Review: Mitochondria as a Target of Environmental Toxicants” it is noted that:
“One well-studied example is adriamycin (doxorubicin), a chemotherapeutic whose clinical use is limited by the fact that it also causes irreversible and cumulative cardiomyopathy (Wallace, 2007). It appears to act largely by poisoning mitochondria, both via redox cycling to generate ROS and by inhibiting ATP production via uncoupling of oxidative phosphorylation. Although its antineoplastic function is largely a result of DNA intercalation and topoisomerase II inhibition in the nucleus, a substantial amount reaches mitochondria, where it has a high binding affinity for cardiolipin, a lipid found only in the mitochondrial inner membrane (Mustonen and Kinnunen, 1993).”
It should be noted that, like adriamycin (doxorubicin), fluoroquinolones also cause topoisomerase inhibition and DNA intercalation. In fact, the mechanism of action for fluoroquinolones is the disruption of topoisomerases, enzymes that are necessary for DNA and RNA replication. (The “Mechanism of Action” for cipro/ciprofloxacin, as listed on the warning label, is “The bactericidal action of ciprofloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV (both Type II topoisomerases), which are required for bacterial DNA replication, transcription, repair, and recombination.”)
Also like adriamycin (doxorubicin), fluoroquinolones bind with and damage cardiolipin, a mitochondrial (and bacterial) membrane lipid. I suspect that the damage done to mitochondrial cardiolipin by fluoroquinolones is key to understanding fluoroquinolone toxicity.
Cardiolipin and Fluoroquinlones
In order to explain the relationship between fluoroquinolone induced damage of mitochondrial cardiolipin and FQ toxicity, I’m going to attempt to summarize and synthesize information from The Journal of Cell Biology, “Making Heads or Tails of Phospholipids in Mitochondria” and the Journal of Medicinal Microbiology, “Comparison of the effects of subinhibitory concentrations of ciprofloxacin and colistin on the morphology of cardiolipin domains in Escherichia coli membranes.” I highly recommend that you read the articles yourself though, because there is a lot of information in them that I won’t be able to get to, and, frankly, I may be wrong in my interpretations of some of the information in the articles.
It should be noted that very little is known about mitochondrial phospholipids. We know how to mess them up through pharmaceuticals and environmental toxins, but we don’t know how to fix them or even detect the damage done to them very well. It is noted in “Making Heads or Tails of Phospholipids in Mitochondria,” which was published in 2011, that, “Although an increasingly detailed structural and mechanistic picture is emerging for the biogenesis, sorting, and compartmentation of mitochondrial proteins (Schmidt et al., 2010), much less is known about mechanisms regulating the supply of phospholipids and the maintenance of mitochondrial membrane integrity.” (Emphasis added) It is also stated that, “The biosynthesis of PE (phosphatidylethanolamine) and CL (cardiolipin) occurs, at least in part, within mitochondria and relies on an intricate exchange of precursor forms between the membrane of the ER (endoplasmic reticulum) and the mitochondrial outer membrane at distinct contact sites, whose structural basis we are just beginning to understand.” And, “Specific mechanisms must exist to ensure the transport of phospholipids from the ER to mitochondria and between outer and inner mitochondrial membranes. However, we are only beginning to understand how these transport processes occur and how they are regulated.” So don’t feel confident at all that even scientists who publish in the Journal of Cell Biology really have any solid answers about how fluoroquinolones, or any other pharmaceutical, mess up mitochondrial membranes.
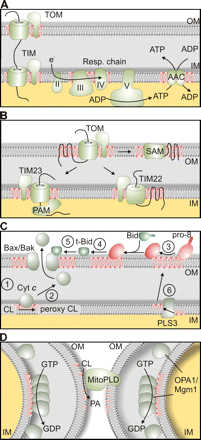
What is known about cardiolipin (CL) is that it creates “tension in membranes that is likely of functional importance to various mitochondrial processes like membrane fusion or the movement of proteins or solutes across membranes,” and “CL regulates multiple steps of the apoptotic program” (apoptosis is programmed cell death) and CL also plays an important role in protein import into mitochondria. Additionally, these pictures (taken directly from “Making Heads or Tails of Phospholipids in Mitochondria,” and all credit should go to the authors and publisher) further explain the important role of cardiolipin:
“The role of CL in mitochondrial processes. (A) CL (depicted in red) affects mitochondrial energy production and is required for dimerization and optimal activity of the AAC and the formation of respiratory chain supercomplexes. (B) Assembly and activity of protein translocases in the outer (TOM) and inner membrane (TIM22 and TIM23 complexes), the SAM complex in the outer membrane, and the assembly of TIM23 complex with the mitochondrial import motor (PAM complex) is supported by CL. (C) Various roles of CL during apoptosis. (1) Cytochrome c (Cyt c) binds to CL in the inner membrane. (2) Release of cytochrome c upon oxidation of CL. (3) Pro–caspase-8 (pro-8) binds to the surface of mitochondria, oligomerizes, and undergoes autocatalytic processing in a CL-dependent manner. (4 and 5) Bid cleavage to truncated Bid (t-Bid) by pro–caspase-8 (4) and activation and oligomerization of Bax/Bak is stimulated by CL (5). (6) PLS3 allows export of CL from the inner to the outer mitochondrial membrane. (D) CL affects fusion of mitochondrial outer and inner membranes. The phospholipase MitoPLD converts in trans CL into PA (depicted in red), triggering the fusion of outer membranes. CL in the inner membrane stimulates oligomerization and GTP hydrolysis of short Mgm1/OPA1 isoforms. IM, mitochondrial inner membrane; OM, mitochondrial outer membrane.”
Got it? Yeah, this stuff is hard. Cardiolipin and the other mitochondrial membranes are important for cellular functioning, and messing up mitochondrial DNA messes up mitochondrial membranes – that’s the gist of it.
Shape Shifting Mitochondria
In “Comparison of the effects of subinhibitory concentrations of ciprofloxacin and colistin on the morphology of cardiolipin domains in Escherichia coli membranes” it was found that “exposure of bacteria to ciprofloxacin significantly increased the percentage of filamentous cells with altered morphology of the cardiolipin domains.” This finding was surprising to the researchers who authored the study because fluoroquinolones are not supposed to work by disturbing the cell wall of bacteria, they disrupt bacterial DNA reproduction, “but surprisingly, changes were found exclusively in CIP-treated cells.” (CIP stands for ciprofloxacin.) They noted that:
“We are not the first to report that DNA topoisomerase inhibition can be followed by the alterations at the level of the bacterial membrane. Dougherty & Saukkonen (1985) showed that inhibition of DNA synthesis by nalidixic acid, a DNA gyrase inhibitor, results in morphological changes consistent with a loss of membrane integrity and leakage of intracellular components. Similar results were presented by Wickens et al. (2000), who noticed a decrease of both membrane integrity and membrane potential after exposure of E. coli to CIP. One of the proposed explanations of this finding is that, as a result of processes induced by inhibition of DNA replication, cells lose their capacity to synthesize necessary components and to maintain the proper membrane structure (Dougherty & Saukkonen, 1985).”
It has been known, SINCE 1985, that fluoroquinolones (the backbone of all fluoroquinolones is naladixic acid) cause “a loss of membrane integrity and leakage of intracellular components.” Intracellular components, especially minerals, especially magnesium, are pretty important. Thanks, FDA, and all the other powers that be within the medical/pharmaceutical complex for completely ignoring that valuable piece of information when making use guidelines for fluoroquinolones. Sigh.
It is also noted in “Comparison of the effects of subinhibitory concentrations of ciprofloxacin and colistin on the morphology of cardiolipin domains in Escherichia coli membranes” that:
“It has also been reported that the unique chemical structure of CL (cardiolipin) allows for trapping of protons and lateral shuttling of them from oxidative phosphorylation complexes to ATP synthase (Haines & Dencher, 2002). This ability determines a unique role of CL as a proton trap within membranes that conduct oxidative phosphorylation and thus ATP synthesis, which suggests why CL is found in membranes which pump protons, e.g. mitochondrial inner membrane and cell membrane of eubacteria (Haines & Dencher, 2002). This specific CL function allows us to speculate that presumably after the fluoroquinolone treatment bacterial membranes maintain CL domains, but (due to membrane perturbation) in altered shapes. Such an effect was observed in our experiments, as CIP treatment induced a significant increase in the number of filamentous cells with FT2 morphology.”
The changing of the shape of mitochondria that is induced by ciprofloxacin induced damage of cardiolipin (and other mitochondrial lipids) is important as well. In the post on Hormones Matter entitled “Hormones, Hysterectomy and the Aging Brain” it is noted that:
“I just finished writing an extensive paper on acquired mitochondrial illness. Over the course of the research, I stumbled upon a short essay linking mitochondrial structure and function to estradiol. More specifically, the rapid estradiol decline common post oophorectomy (ovary removal), fundamentally alters the shape, and ultimately, the function of mitochondria. (emphasis added) Researchers found that a rapid decline in estradiol evokes significant damage in the brains (and presumably other body parts) of female monkeys. Additional studies using estradiol starved mitochondria from female rodents showed similar shape alterations and consequent declines in brain bioenergetics. Interestingly though, with natural menopause, where estradiol declines more gradually, no such structural changes were observed. In fact, with the more gradual decline in estradiol the mitochondria appear to increase their production of the lifesaving ATP as a compensatory reaction.”
Mitochondrial shape, which is determined by phospholipid integrity, is connected to hormones and brain health as well. It’s all connected. Fluoroquinolones throw a wrench in the whole thing.
Molecular Shape of Mitochondrial Lipids and Possible Solution
It is noted in “Making Heads or Tails of Phospholipids in Mitochondria” that “The non–bilayer-forming lipids PE and CL are more conical shaped with a smaller hydrophilic head group diameter and a relatively larger hydrophobic domain diameter. This shape allows the formation of hexagonal phases that can be observed for isolated lipids depending on the pH and ionic strength” and that, “Early studies with model membranes demonstrated that the formation of hexagonal structures induce membrane fusion and suggested a crucial role of non-bilayer lipids such as CL or PE for membrane fusion in vivo.”
This may be a reach and I could be wrong to connect these things, but the mention of a hexagonal structure made me think of a treatment that has helped several long-time floxies. The treatment is called Buckminsterfullerene/c60. In the few articles that I’ve read about Buckminsterfullerene/c60 it appears that the molecular shape is important. Per the article, “Liposome Formulation of Fullerene-Based Molecular Diagnostic and Therapeutic Agents,” published in Pharmaceutics, “Fullerenes are hollow cage molecules with sp2 carbon atoms arranged in hexagons and pentagons.” They’re also powerful antioxidants and the results that the floxed friends are getting from supplementing with Buckminsterfullerene/c60 are impressive.
I think that a lot more research needs to be done on Buckminsterfullerene/c60 before it is determined to be an effective or safe treatment for FQ toxicity. As with everything, do your own research, be careful, know that I’m not a doctor, etc.
Here are a couple of articles about Buckminsterfullerene/c60:
Huffington Post, “Do Buckyballs Extend Lifespan?”
Vaughter Wellness, “Aerobic- & strength gains, longevity, brain rejuvenation and tumor prevention with lipofullerene C60 olive oil”
It is also noted in “Fullerenes are hollow cage molecules with sp2 carbon atoms arranged in hexagons and pentagons” that, “Fullerene medicine is a new but rapidly growing research subject. Fullerene has a number of desired structural, physical and chemical properties to be adapted for biological use including antioxidants, anti-aging, anti-inflammation, photodynamic therapy, drug delivery, and magnetic resonance imaging contrast agents.”
I don’t currently have enough information to say whether or not Buckminsterfullerene/c60 will help to put your mitochondrial lipids (especially cardiolipin) back together.
But I do think that there is enough information about the severe damage done by fluoroquinolones to mitochondrial lipids that another black box warning for mitochondrial toxicity is warranted.












The PK Protocol may also help to put mitochondrial lipids back together – http://www.pkprotocol.com/
Hi Lisa, I first would like to thank you for all of the hard work that you do to give Floxies hope! I was wondering if you had any additional information regarding what symptoms were improved with the Floxies who took the c60? Or any info on their protocols, source, or length of time that they took the c60? Thanks again!
Sent from my iPhone
>
Reblogged this on The Other Side Of The Stretcher and commented:
The Foxie Hope Blog has a very good post about MITO Toxicity Caused by Flouroquinolones Antibiotics!
I want to leave a strong warning note about C60. Half a year ago I had an adverse reaction to ciprofloxacin. I had mild physical problems, but severe cognitive and emotional problems – psychosis, fear, brain fog/depersonalisation, memory loss. I was prescribed antipsychotics and benzodiazepines, both of which led to a severe increase in symptoms.
Recently I started to recover a little bit. My energy and concentration levels went up, my fear went down and now and then I could be a little bit happy and loving again.
I decided to try one dose of C60 to see if it could help my recovery.
After one hour I went outside and noticed a rapid increase of all my symptoms. Fatigue, brain fog/depersonalisation, weird headaches, stomach problems, nausea, etcetera. It has been 5 days now and my symptoms are not getting better, they even seem to get worse. There seems to be a negative response to sunlight and to food.
I have posted his to Longecity forum and another user there had a similar return of mental floxing symptoms after C60 use, in combination with sun exposure.
Please take care!
This is an extremely experimental drug and not much is known about it. Side effects and interactions are still not known and tested.
I recently found out that one of the reasons I’ve been getting deathly sick after eating fats and proteins has been due to severe carnitine deficiency, required for transporting fats into the mitochondrial. Without carnitine, the fats accumulate, causing organ damage.
Fullerine is a very long-chain lipid (60 carbon backbone), so it may require substantially more carnitine for transport into the mitochondria.
I used to suffer severe reactions to coenzyme Q10, which I am deficient in, until I learned about the carnitine and started supplementing with acetyl L-carnitine.
Adverse reactions to fats and lipid-based supplements could be a sign of carnitine deficiency.
This article fascinated me! My husband was poisoned by FQs mutiple times between 2009-2017.. Cipro was right after a 6 month stay in the hospitals.. horrible horrible drugs!! How do we get them off the market??
He is doing well.. but it’s a career keeping him good! NrF1 and NrF2 from LifeVantage are the reason he survived!! Survival genes .. wake them up!!
I am suffering seizure/panic attacks, heart palpatations and breathing problems as a result of taking Ciplox antibiotics which contain fluoroquinolones. I have had 17 seizures/panic attackes since 6th Septemeber 2019. Feeling desparate, frightened and exhausted and under the care of a Neurosurgeon with costly accounts been settled without medical aid help. Feeling desparate!
I would love to keep hearing from people who have negative and positive stories and information as we are all in this together for the same reason. Every little bit of information can give us hope for a healthy body so we can enjoy our lives we all so deserve to in this toxic world.
… [Trackback]
[…] There you can find 37309 more Info on that Topic: floxiehope.com/fluoroquinolones-damage-mitochondrial-lipids/ […]
… [Trackback]
[…] Read More here to that Topic: floxiehope.com/fluoroquinolones-damage-mitochondrial-lipids/ […]
… [Trackback]
[…] Read More Info here on that Topic: floxiehope.com/fluoroquinolones-damage-mitochondrial-lipids/ […]
… [Trackback]
[…] There you will find 55296 additional Information to that Topic: floxiehope.com/fluoroquinolones-damage-mitochondrial-lipids/ […]
… [Trackback]
[…] Find More Information here on that Topic: floxiehope.com/fluoroquinolones-damage-mitochondrial-lipids/ […]